About Luminopia
Luminopia is the first FDA Approved, VR healthcare binocular therapy for treating amblyopia in children ages 4-7. Amblyopia, or lazy eye, is a neuro-visual disease where patients have significant vision loss in one eye caused by a deficit in the visual pathway in the brain. It affects 3% of all children and 2% of adults, making it the leading cause of vision loss in kids. If left untreated, amblyopia can lead to permanent vision loss and increase the risk of lifetime blindness.
Traditional treatments, such as eye patching and atropine drops, leave significant unmet needs for patients. First, patching and atropine drops punish the stronger eye, and don’t teach the eyes how to work together properly. Second, compliance with patching and atropine is notoriously difficult, and patching can come with a harmful social stigma especially for children. Even when patients are compliant, one nationwide study found that 75% of patients still had significant vision loss after going through patching.
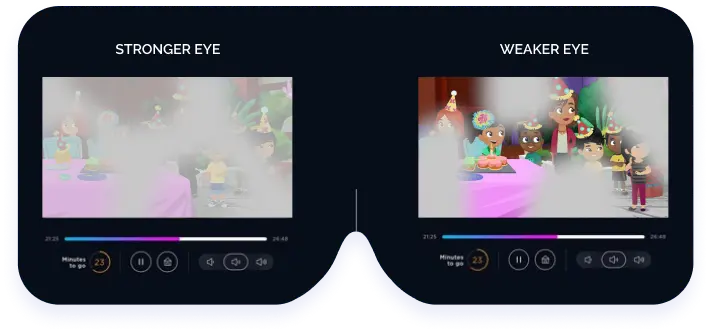
Luminopia’s VR healthcare solution is simple, engaging, and effective. Luminopia’s treatment allows patients to watch TV for 1 hr per day, 6 days per week in VR. Their proprietary software uses a dual-action mechanism which modifies visual content delivered in a VR headset and presents it differently to each eye to rebalance the input to the brain. This treatment teaches patients to use both eyes together. Compliance with Luminopia was around double that of patching in their clinical trials. Children can experience significant vision improvement within a month.
VR Healthcare Solution Development and FDA Approval Process
Luminopia has demonstrated efficacy and safety across 3 Trials, at 23 sites in over 200 patients; including a gold standard, randomized controlled Phase 3 trial which demonstrated the safety and efficacy of the therapy for children aged 4-7 years. Their solution has been validated through a similar development process as a new pharmaceutical product.
Embarking on this rigorous process required the Luminopia team to scale their VR solution to hundreds of patients all across the country. During the commercialization phase, Luminopia partnered with ManageXR to solve the challenges that arose when scaling their VR device fleet. Hurdles such as remote management, security and privacy, and ease of use became mission critical to ensuring an excellent and effective patient experience.
Luminopia Scales Successfully with ManageXR
ManageXR’s device management platform solved the challenges of scaling and commercializing Luminopia’s VR solution to achieve FDA Approval. Luminopia uses ManageXR’s VR and AR MDM to secure devices, protect patient PII, remotely manage hundreds of devices, prototype automations, and deliver a seamless and intuitive VR experience for patients who have never used VR before.
Deliver a seamless and intuitive patient experience
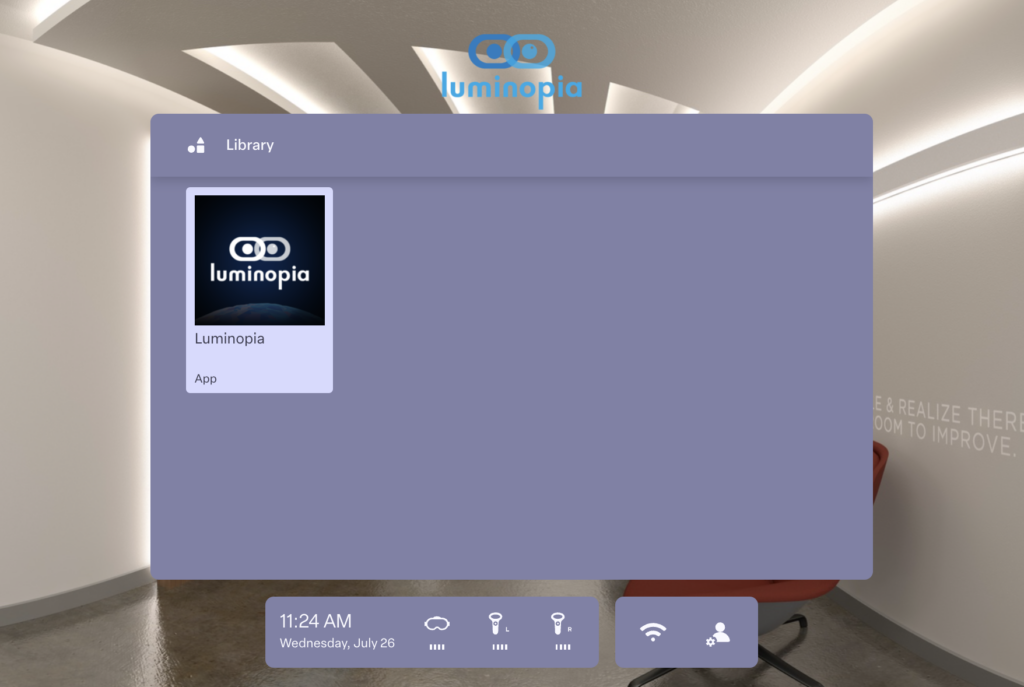
Patients need to access therapy easily and quickly when the VR device arrives at their homes. ManageXR’s Custom Home Screen makes it possible for Luminopia to simplify the in-headset experience, transforming a consumer device into a therapy-ready tool. Luminopia successfully deployed their VR devices to 200 patients without the need for any technical support. From Luminopia’s branded Custom Home Screen environment, patients can only access their approved and prescribed treatment. The locked down experience prevents kids from accessing unapproved apps, websites, social media, and advertisements while also simplifying the immersive environment.
Secure and locked down VR devices
Luminopia leverages ManageXR’s device security settings to ensure that their devices are always protected. ManageXR is SOC 2 Type 2 certified and has all the critical security features to ensure devices are safe and secure.
- Factory reset to protect data and IP
- Disable user factory reset
- Disable developer mode
- Disable USB File Transfer
- Lock access to the device with a password
- Customizable user roles
- User audit logs
Protect patient information
When it comes to patient care and healthcare compliance, privacy is integral. Luminopia is able to hide user-identifying information (device location, mac address, wifi network name) on ManageXR to protect patient PII.
Remotely manage hundreds of VR devices with ease
Configurations give Luminopia the control they need as they scale their fleet and impact. The team can easily manage all of their devices using ManageXR’s simple and centralized dashboard. They can quickly determine which devices are with patients, verify that the devices are healthy, and monitor utilization.

Accelerate workflows with the ManageXR API
Using the API, Luminopia can select a batch of devices from a list of serial numbers, then efficiently apply tags and configurations to them, all from Luminopia’s own internal tools.
ManageXR is the VR MDM for Healthcare
ManageXR was founded in healthcare and is built specifically for VR and AR devices. Our team understands the requirements and standards for working in healthcare and through the FDA Approval process for pharmaceutical solutions. ManageXR is secure, protects patient privacy, and is easy for patients to use. Our platform has many capabilities uniquely needed for VR devices, like Custom Home Screen. With ManageXR’s VR device management platform backing their solution, Luminopia can focus on delivering life changing vision therapy to children and adults all across the country.